(Mentors: Marc-Jan Gubbels, Prof. of Biology; Eranthie Weerapana, Assistant Prof. of Chemistry)
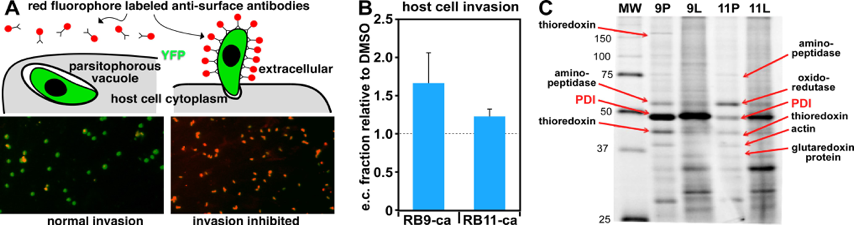
Description: The apicomplexan organism Toxoplasma gondii utilizes fast rounds of lytic intracellular replication cycles. Identifying proteins essential to the various stages of Toxoplasma infection will provide a better understanding of the underlying molecular mechanisms that are active in this organism. In this project, the expertise of the Gubbels Lab and the Weerapana Lab come together to identify proteins essential to host cell invasion. In preliminary studies, the Gubbels Lab screened a library of cysteine-reactive small molecules developed in the Weerapana Lab in phenotypic assays to identify compounds with significant effects on Toxoplasma host cell invasion. From this screen, two bioactive compounds, RB-9-ca and RB-11-ca, significantly impaired Toxoplasma host cell invasion (Figure 3). Mass spectrometry-based proteomics methods identified the protein target of these compounds as Toxoplasma protein disulfide isomerase (TgPDI). The future goals of this collaborative project are two-fold. First, the role of TgPDI will be probed by generating parasite mutants with an optimized ER retention motif, which should have a lowered invasion capacity according to the posited model. In turn, supplementation of these mutants by exogenous recombinant PDI should then rescue the phenotype, while the same experiment on wild type parasites should boost invasion efficiency. The second goal is to improve the potency and selectivity of cysteine-reactive probes for TgPDI. To this end, a structure-activity relationship around the lead compound, RB9-ca, will be established through the synthesis of targeted libraries. These second-generation inhibitors will be assessed using in vitro enzymatic assays for potency against recombinant TgPDI versus human PDI, and in cells for selectivity across the Toxoplasma and human proteome. The optimized inhibitor(s) generated from these studies will be evaluated for effects on host cell invasion, and to further mechanistically elucidate the endogenous role of TgPDI.Together, these studies will provide new insights in the underlying mechanism of Toxoplasma.